In today’s rapidly evolving diagnostic landscape, Lab Developed Tests (LDTs) play a vital role in advancing healthcare innovation. Designed, validated, and performed entirely within a single laboratory, these tests enable providers to deliver faster, more specialized results that traditional analysis may not offer. As precision medicine continues to grow, LDTs are helping laboratories meet unique clinical needs with flexibility, accuracy, and speed.
What Are Lab Developed Tests?
A Lab Developed Test (LDT) is an in-house diagnostic analysis created and validated by a clinical laboratory for its own use. Unlike commercially distributed in-vitro diagnostic (IVD) kits that undergo FDA premarket approval, LDTs are designed and controlled under CLIA (Clinical Laboratory Improvement Amendments) regulations. This gives laboratories the ability to develop new methodologies and respond quickly to emerging medical needs.
The Value of LDTs in Clinical Practice
LDTs often fill diagnostic gaps where no FDA-cleared test exists or where current options are insufficient. They are used across a wide range of specialties—including oncology, reproductive health, infectious disease, and genetics—to deliver results that are:
- Highly Specialized: Tailored to specific biomarkers, patient populations, or niche diagnostic needs.
- Rapidly Deployable: Developed and validated quickly in response to new medical findings or public health demands.
- Flexible and Customizable: Adapted to new technologies and evolving scientific standards.
This adaptability makes LDTs invaluable to physicians and researchers seeking timely, actionable data to guide treatment planning and improve patient outcomes.
Regulatory Considerations
While LDTs are regulated under CLIA, discussions around FDA oversight continue to evolve. Laboratories must maintain rigorous validation, documentation, and quality-control procedures to ensure accuracy, reliability, and patient safety. As the industry moves toward greater standardization, maintaining compliance remains essential to preserving both innovation and trust.
LDTs and the Future of Fertility Diagnostics
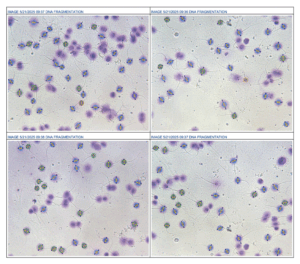
In male and female fertility testing, LDTs enable advanced analyses—from sperm DNA fragmentation and oxidative stress to hormone profiling—that may not yet be available through commercial kits. These specialized analyses allow fertility labs to provide deeper insights, improve clinical decision-making, and support more personalized reproductive care.
Driving Innovation in Diagnostics
Ultimately, Lab Developed Tests empower laboratories to push the boundaries of diagnostic science. By combining scientific creativity with clinical responsibility, LDTs will continue to shape the future of precision medicine—helping clinicians deliver faster answers, more accurate results, and better outcomes for every patient.